Lateral flow assays (LFAs), also known as immunochromatographic assays or rapid tests, are fast, easy-to-use and portable devices that can detect the presence of a target substance in a liquid sample without the need for specialised staff and expensive equipment. These tests are widely used in medical diagnostics for point of care testing (e.g. home testing) or laboratory use. Because of their compact size and reduced cost, LFAs are often referred to as Point-Of-Care Tests (POCT). The most common example of LFA is probably the home pregnancy test.
Structure of a LFA test
The concept behind Lateral flow assay is simple: a liquid sample (or extract) comprising the analyte of interest moves across many zones of polymeric strips, without the help of external forces (capillary action), where the molecules that interact with the analyte are attached to the polymeric strips.
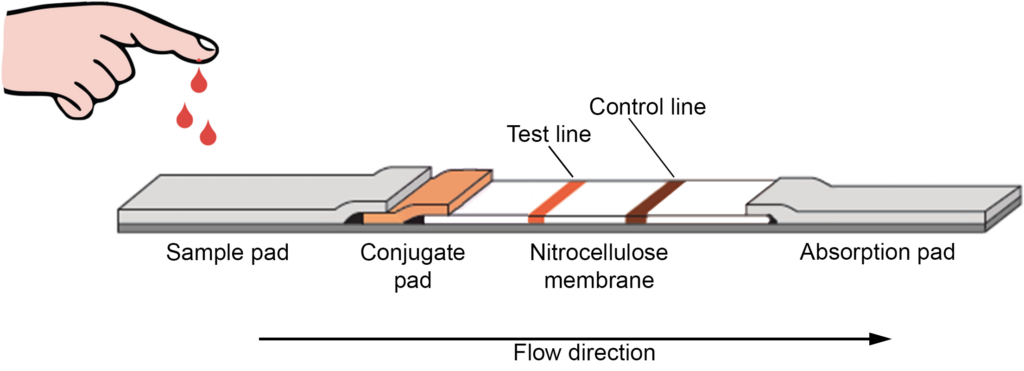
A standard lateral flow test strip comprises of overlapping membranes placed on a backing card to improve stability and usability. As illustrated in the figure, the sample can be impregnated with buffer salts and surfactants on the adsorbent sample pad at the one end of the strip, making the sample appropriate for interaction with the detective system.
The sample pad makes sure that the analyte in the sample may bind to the conjugation reagents and to the membrane. The processed material migrates via conjugate release pad which includes antibodies that are attached to coloured or fluorescent fragments—most frequently colloidal gold and latex microspheres. The sample migrates down the strip to the detection zone with the conjugated antibody attached to the target analyte.
This is a porous membrane with certain biological components (most often antibodies or antigens) that are immobilised in lines, and which generally consists of nitrocellulose. Its job is to react to the conjugated antibody bound with the analyte. The sample analyte recognition results in an adequate response on the test line, while the control line response shows the correct liquid flow by the strip. The read-out can be evaluated either by an eye or by means of a specialized reader, represented by lines with varying intensities. Additional test lines containing antigens specific to other analytes can be immobilized in an array format to test several analytes simultaneously under similar conditions.
On the other hand, the semi-quantitative assays can be carried out using several test lines loaded with the same antibody. The concept behind this ‘ladder bar’ assay is that the immobilized antibody complexes are gradually captured on each successive line by the colorimetric conjugate-antigen complexes where the number of lines on the band is directly related to analyte concentration. The liquid passes through the device due to the capillary strength of the strip and an absorbent pad is placed at the end of the strip to sustain this motion. The absorbent pad has a function to wick the chemical reagents and prevent liquid backflow.
A typical lateral flow device is represented in the image below, and typically includes the following components:
- Sample pad.
- Conjugate pad, where the labelling reagent (e.g. antibody-conjugated EasyConjTM quantum dots) is dried. The labelling reagent should be released rapidly when the sample comes into contact.
- Nitrocellulose membrane, where the antibodies are striped. The membrane is often laminated onto a plastic backing.
- Wick/Absorbent Pad, controls the sample flow along the strip.
- Plastic housing (cassette).
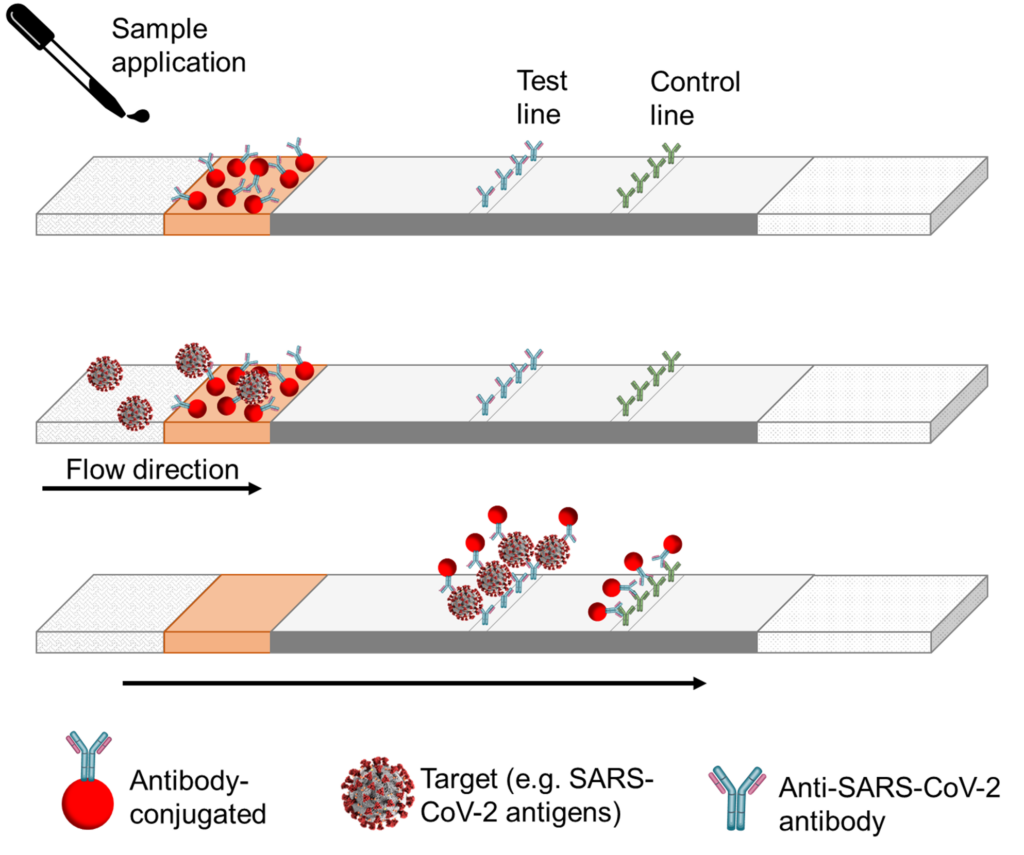
During a typical lateral flow test in a ‘sandwich’ format (see image below), the sample is added to the sample pad, the sample is added to the sample pad, then flows through to the conjugate pad, where releases the dried labelling reagent. The target and the labelling reagent start to mix and form a complex, migrating towards the antibodies immobilised on the nitrocellulose membrane. These immobilised antibodies capture the target-label complex, allowing for the formation of a line. If the target is present, a line will form where the test line is; if the target is not present (or below the limit of detection of the assay), a line will not form. The control line should always appear, as it signals that the sample and the reagents are flowing correctly. Any unreacted labels and excess reagent past the capture zone to the absorption pad.
The wicking rate is one of the most important characteristics of a nitrocellulose membrane, since it affects the reaction time between the antibodies and the target. Typically, the slowest the wicking rate, the more sensitive is the test.
Labels for LFA tests
Various types of labelling reagents can be used for the visualisation of the lines. The most commonly used material are gold nanoparticles (‘colloidal gold’) and latex beads. However, other labelling reagents have been developed in recent years, including enzyme conjugates, magnetic particles and fluorescent particles – such as quantum dots.
Advantages of LFA tests
The lateral flow technology is particularly useful for the production of rapid and portable point-of-care tests. These tests have made a significant contribution to reducing the spread of infectious diseases, one recent example being COVID-19.
- Low sample volume, low cost and rapid
- One-step assay with no washing steps necessary
- Easy to use and simple test procedures to follow
- Qualitative or semi-quantitative results
- Hormones, proteins, nucleic acids, haptens, amplicons can be detected
- No refrigeration required, long shelf life and can be prepared in batches in advance
- Applications enter the market faster with relatively short development time
References
- Merk Millipore. Rapid Lateral Flow Test Strips – Considerations for Product Development. 2013 (link)

