
Moungi G. Bawendi, Louis E. Brus and Alexei I. Ekimov have been awarded the Nobel Prize in Chemistry 2023 for the discovery and development of quantum dots.
Introduction
Quantum dots (QDs) are semiconductor nanoparticles with size-dependent optical and electronic properties. They usually measure from 2 to 10 nanometers in size and they have unique optical properties, including wide absorption and narrow emission spectra. One of the most notable properties of quantum dots is the ability to change the wavelength of their fluorescence emission by changing the nanoparticle size – as the size of the particle increases, the colour of the emitted light shifts from blue to near-infrared.
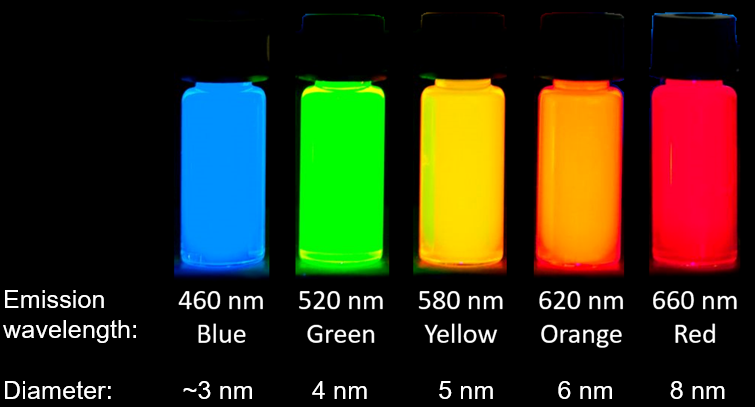
Quantum dots that emit in the visible region of the electromagnetic spectrum are often made of CdSe. However, CdSe QDs are often coated (i.e. ‘passivated’) with shells of CdS and/or ZnS. These nanoparticles are called ‘core-shell quantum dots’ and show greatly improved stability and higher fluorescence intensity compared to CdSe particles.
Quantum dots are typically prepared in organic solvents, which make them insoluble in water and thus not suitable for biological application. Therefore the surface of core-shell quantum dots needs to be modified to become hydrophilic, usually using amphiphilic molecules or polymers. As a result, the quantum dots can be dispersed in water, a process often called “phase transfer”.(Medintz, 2005)
Quantum dots have significant advantages over common fluorophores, including better photostability, narrow optical emission spectra and long shelf-life. Quantum dots are also very bright, where the ‘brightness’ is defined as the product of the molar absorption coefficient at the excitation wavelength and the fluorescence quantum yield (i.e. the number of emitted photons occurring per number of absorbed photons).(Resch-Genger, 2008)
Quantum dot absorption and emission spectra
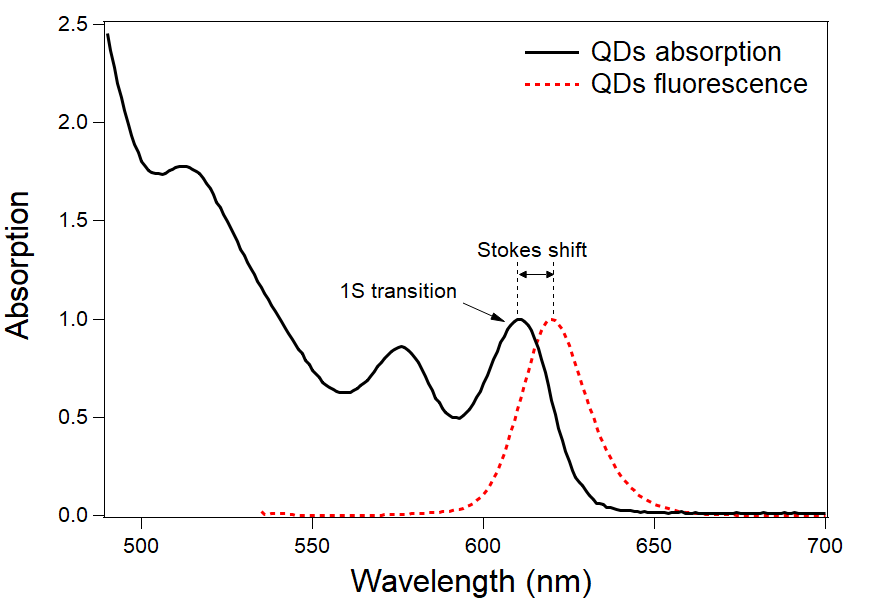
The quantum dots are particularly efficient at absorbing UV light, with their extinction coefficient increasing dramatically below 500 nm. The absorption spectrum has also some distinctive features, such as the “1S transition”, that define the theoretical Stokes shift of the particle.
The emission spectrum of quantum dots are narrow and symmetrical, which make them ideal for multiplexing applications.
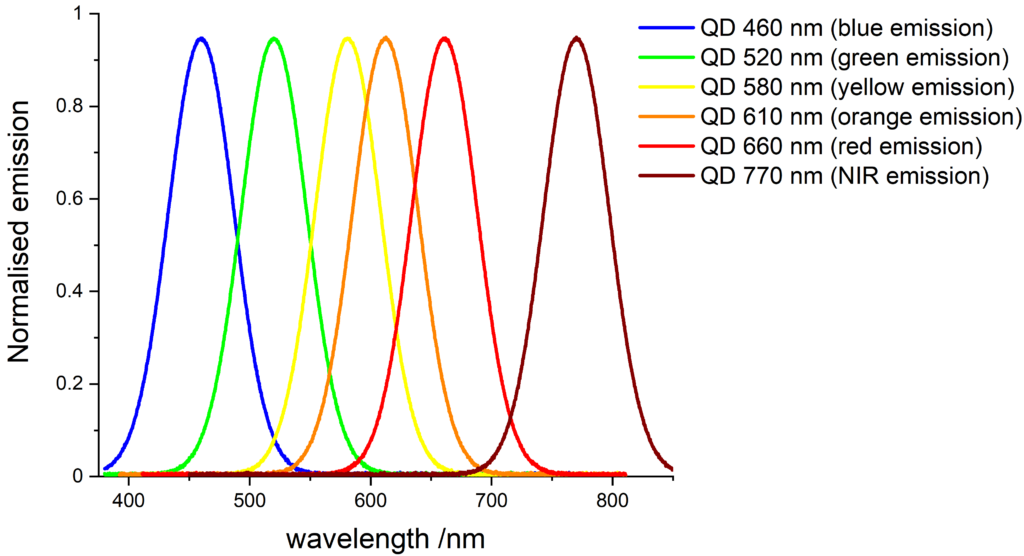
Quantum dots can replace traditional organic dyes in most applications:
- The emission of blue-emitting (460 nm) EasyConjTM quantum dots is equivalent to Pacific Blue dye.
- The emission of green-emitting (520 nm) EasyConjTM quantum dots is equivalent to fluorescein (FITC) and Rhodamine 110 dyes.
- The emission of yellow-emitting (580 nm) EasyConjTM quantum dots is equivalent to tetramethylrhodamine (TAMRA, TRITC) and R-phycoerythrin (R-PE) dyes.
- The emission of orange-emitting (610 nm) EasyConjTM quantum dots is equivalent to Texas Red dye.
- The emission of red-emitting (660 nm) EasyConjTM quantum dots is equivalent to Cy5 and Nile Blue dyes.
- The emission of NIR-emitting (770 nm) EasyConjTM quantum dots is equivalent Cy7 dye.
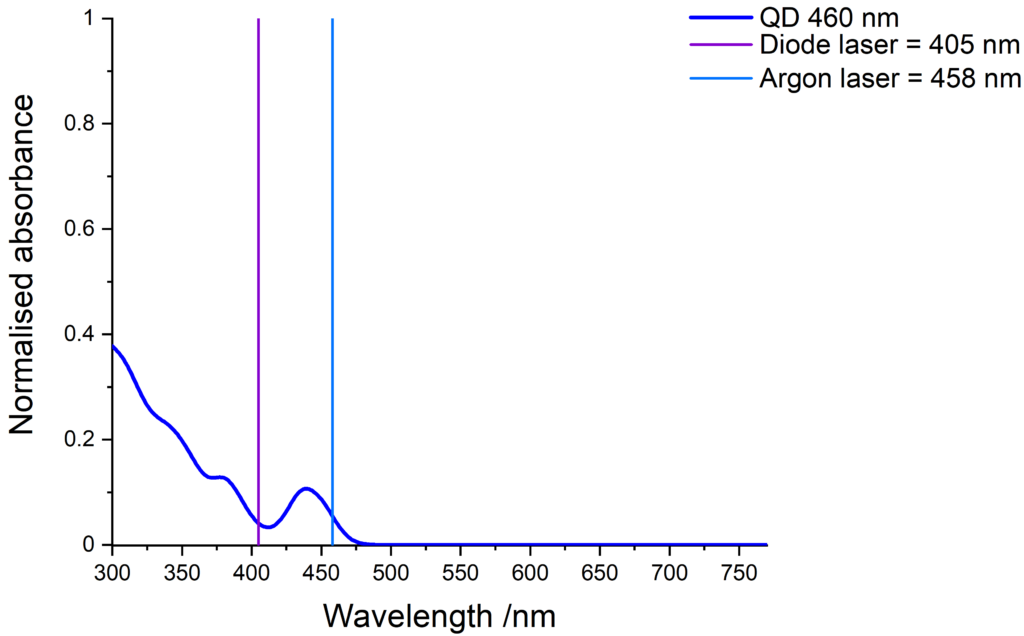
Quantum dots excitation
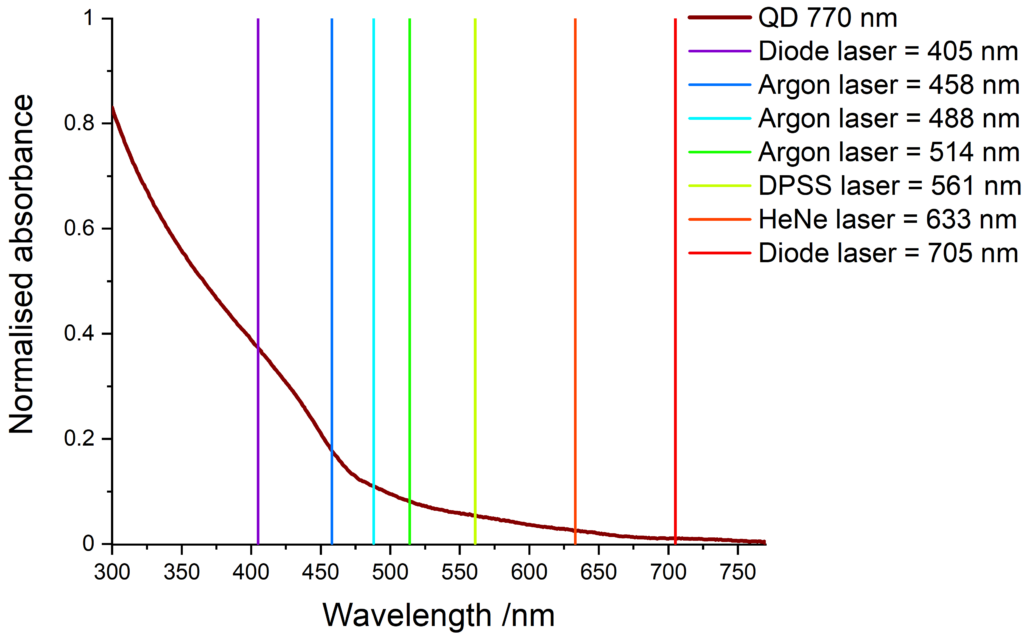
Quantum dots have a wide absorption spectrum and are compatible with various light sources. The images below show some common laser lines that can be use to excite our quantum dots.
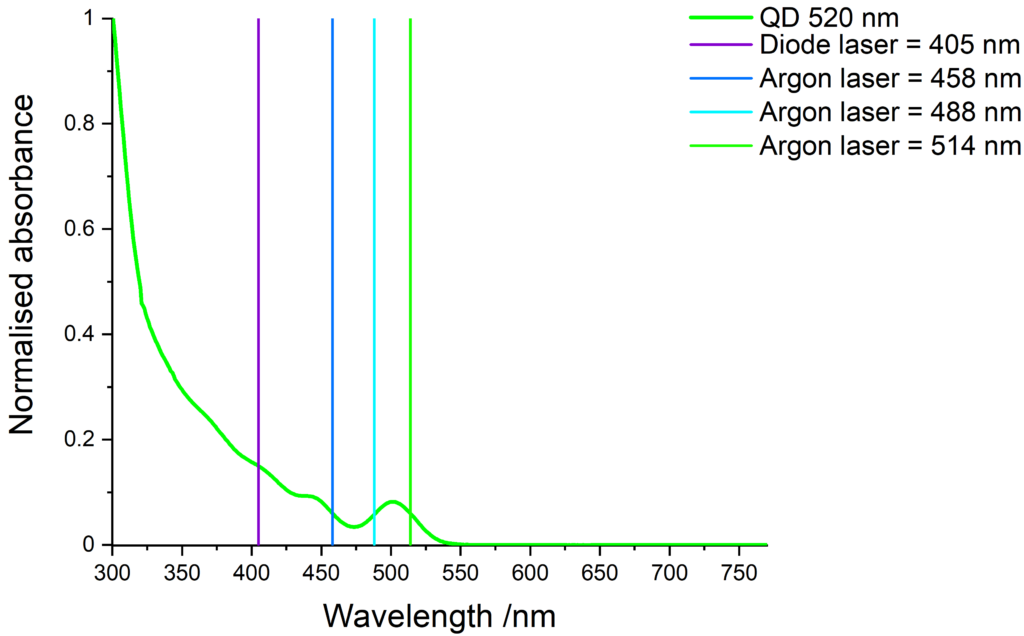
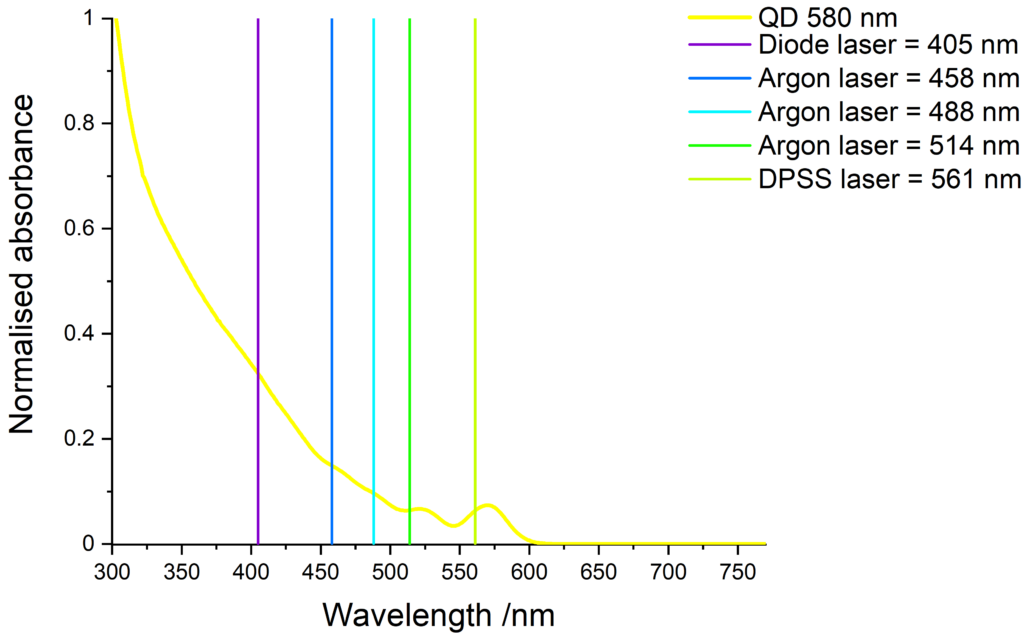
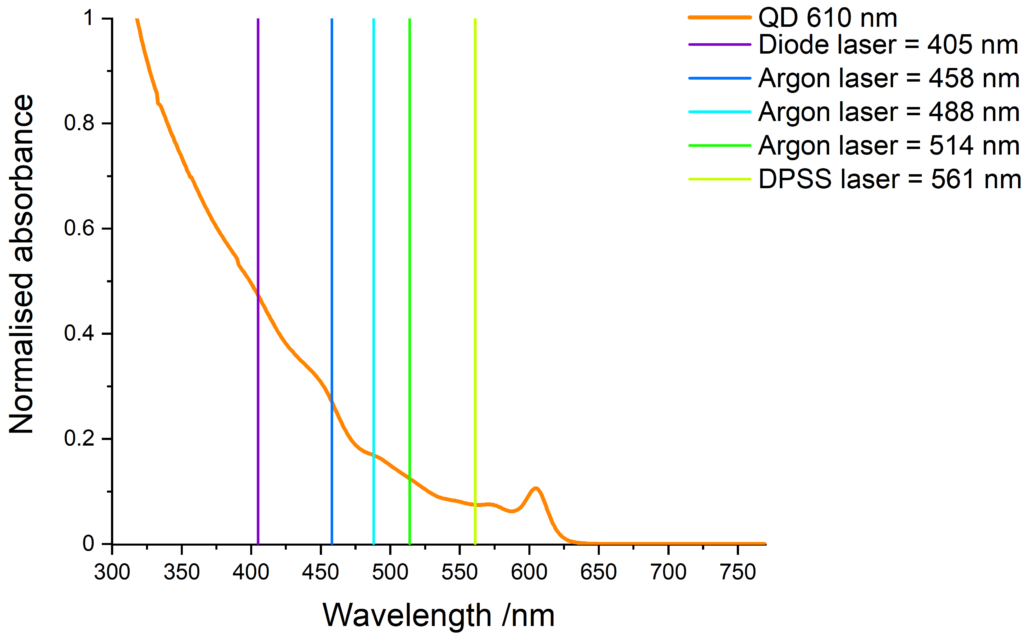
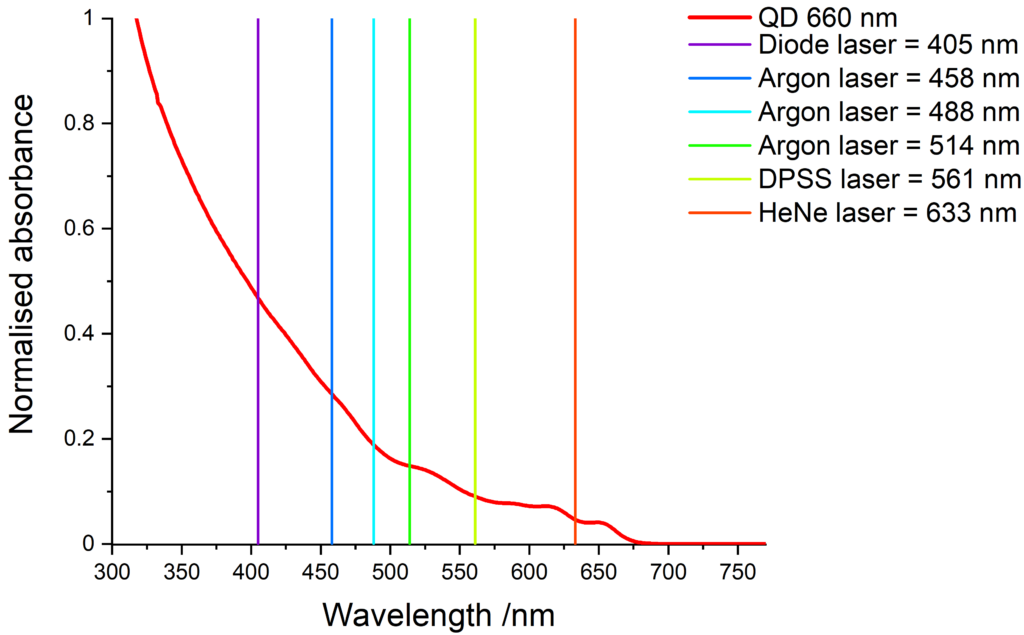
Cd-free quantum dots
CuInS2
References
- I. L. Medintz, H. T. Uyeda, E. R. Goldman, H. Mattoussi. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials, 2005, 4, 435–446 (link)
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke, T. Nann. Quantum dots versus organic dyes as fluorescent labels. Nature Methods, 2008, 5, 763-775 (link)

